Scientists Unravel the Potential of Aqueous Amino Acids for Direct Air Capture of CO₂
Researchers at the Department of Energy’s Oak Ridge National Laboratory have taken a significant step in comprehending a viable approach for direct air capture (DAC) of carbon dioxide from the atmosphere. This DAC process, still in its early development, aims to achieve negative emissions, surpassing the amount of carbon dioxide removed from Earth’s surrounding gases compared to what is emitted.
The recently published research in Cell Reports Physical Science delves into the foundational stages of carbon dioxide sequestration using aqueous glycine, an amino acid recognized for its absorptive properties. Through the application of advanced computational methods, the scientists investigated less-explored dynamic phenomena in liquid solutions pertaining to the rate at which carbon dioxide can be captured.
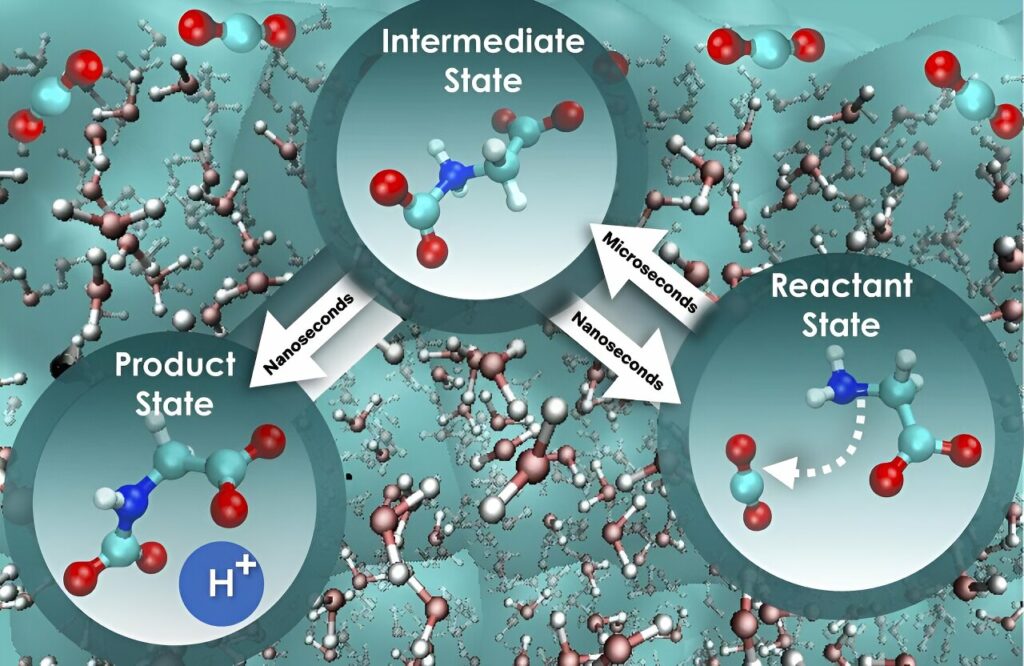
“Chemical reactions in water are intricate, especially when the movement of water molecules is a significant factor,” explained Santanu Roy, who, along with colleague Vyacheslav Bryantsev, designed the computational investigation. “Water molecules and chemicals engage in something akin to a coupled dance that can either marginally or significantly impede the reaction. Understanding these dynamic interactions, known as nonequilibrium solvent effects, is crucial for obtaining a comprehensive understanding of how reactions operate and their speed.”
The researchers found that solely examining the free energy barrier, the energy threshold that must be surpassed for a system to transition between states, when assessing the rate of carbon dioxide absorption is an oversimplified approach that does not present the full picture. This incomplete method may lead to an inaccurate comprehension of reaction kinetics—the factors influencing the speed of a reaction. “We employed a more comprehensive approach that considers the influence of water on the motion along the reaction path, and the results were intriguing,” noted Bryantsev. “
The initial step, where glycine interacts with carbon dioxide, is nearly 800 times slower compared to the next step, where a proton is released to ultimately form a mixture of product states for holding the absorbed carbon dioxide. “Remarkably, the free energy barrier remains constant for both steps, and so this alternative perspective truly distinguishes the speed of these two crucial stages, offering a pathway to enhance the efficiency of carbon dioxide absorption and separation.”
While the extensive ab initio molecular dynamics simulations used in the study had limitations in terms of time and length scales and computational costs, the researchers plan to combine a machine-learning approach with accurate simulations for future projects. This will enable molecular simulations at large scales with significantly reduced computational costs. “While we have illustrated a molecular-level kinetics picture of carbon dioxide capture by aqueous amino acids, utilizing the machine-learning approach will help us comprehend the effects of macroscopic factors such as temperature, pressure, and viscosity on DAC, and how these effects relate to the molecular-level picture,” added Xinyou Ma, who conducted the simulations. The study’s findings illuminate the complex processes involved in DAC, emphasizing the crucial role of kinetics, thermodynamics, and molecular interactions in removing carbon dioxide from the atmosphere using aqueous amino acids.
As these mechanisms are better understood, the feasibility of deploying large-scale DAC technology becomes more realistic. Globally, various DAC projects are in different stages of research, testing, and development.
This article is republished from PhysORG under a Creative Commons license. Read the original article.
Do not forget to share your opinion with us to provide you with the best posts !



0 Comments