Innovative catalytic method accelerates the production of a crucial component in an incontinence drug.
A research group at Nagoya University in Japan has developed a new catalyst that promises to revolutionize the asymmetric synthesis of pharmaceuticals called chiral macrocyclic dilithium(I) salt. It overcomes the lack of reactivity of ketones and the difficulty inducing them to arrange atoms, which are common challenges in drug-making.
The researchers used their technique to synthesize a key intermediate of the incontinence drug oxybutynin. Their catalyst promises to contribute to future drug discovery and development. They published their results in the Journal of the American Chemical Society.
“This research represents a major advance in chiral drug synthesis,” said Professor Ishihara from Nagoya University. “Our catalyst can facilitate the rapid synthesis of complex compounds. This holds great promise for future drug discovery efforts.”
All drugs are made from precursor chemicals. Ideal precursors are versatile compounds that can create a wide variety of end products. One particularly versatile precursor is optically active tertiary propargyl alcohol. It is used to create pharmaceuticals, including anticancer agents, antibiotics, and antivirals.
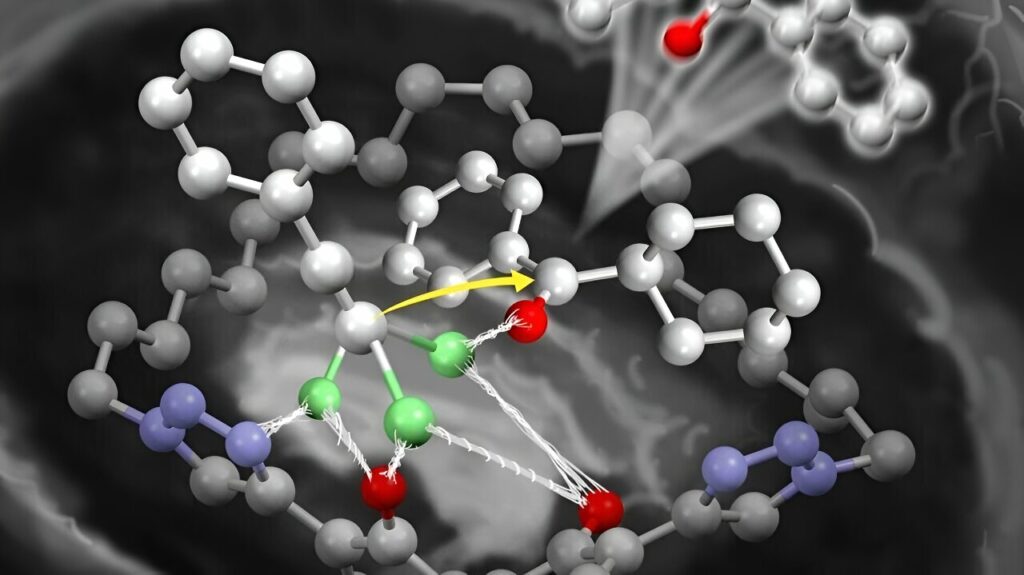
However, production of these important chemicals is hindered by the low reactivity of ketones, which are precursors of tertiary propargyl alcohols. In addition, there is the difficulty of their asymmetric induction, a process that favors the creation of a specific arrangement of atoms that is more suitable than other arrangements for making the drug.
To overcome the low reactivity of the ketones, highly reactive lithium-based reactants, called lithium acetylides, are added. However, their reactivity is often insufficient for use with ketones. The development of a new catalyst was needed to promote the reaction and control the selection of the optimal arrangement of atoms.
Enzymes are ideal for these reactions, as they lower the energy required to make the reaction occur. However, due to their large and complicated structure, the synthesis of enzymes is difficult.
The currently used acyclic dilithium catalyst-based approach was pioneered by Kumamoto University’s Makoto Nakajima. However, this approach has a limited substrate scope due to the self-aggregation of catalysts and an overly long reaction time of up to 12 hours. This creates a bottleneck when producing the desired drug.
Professor Kazuaki Ishihara and his collaborators, which included his graduate students, developed a chiral macrocyclic dilithium(I) salt. It is a simple catalyst that functions like an enzyme, overcoming the decrease in reactivity by activating less reactive ketones.
This allows the addition of acetylides, such as lithium acetylides. The large macrocyclic structure of the catalyst allows them to catalyze even bulky ketones. This prevents aggregation between the catalyst and the lithium-based reactants.
Despite being simpler than enzymes, the researchers found that their catalyst was more efficient than other known catalysts. They successfully synthesized optically active tertiary propargyl alcohol from a variety of ketones. Although this industrial alcohol is difficult to produce by conventional methods, they synthesize it in 5 to 30 minutes. This is much faster than the 12 hours that Nakajima’s catalyst-based production process takes.
The addition of alkynyls to carbonyl compounds, such as ketones, is a valuable synthetic method for the preparation of versatile chiral alcohols that are widely found in pharmaceuticals and natural products. This research is a breakthrough in modern synthetic organic chemistry and a promising leap forward in drug discovery.
This article is republished from PhysORG under a Creative Commons license. Read the original article.
Do not forget to share your opinion with us to provide you with the best posts !



0 Comments